The only pivotal study for PBC in which
40% of patients had baseline ALP >3 x ULN1
The ELATIVE® trial was a double-blind, randomized, placebo-controlled study to evaluate the efficacy and safety of IQIRVO in patients with PBC and inadequate response or intolerance to UDCA.2
- Placebo + UDCA referred to as “UDCA alone”
- 95% (153/161) of patients on IQIRVO in the ELATIVE trial received concurrent UDCA therapy2
- ULN for ALP was defined as 104 U/L for women and 129 U/L for men2


|
Baseline characteristics1 |
IQIRVO + UDCA |
UDCA alone |
OLE crossovera |
|---|---|---|---|
|
Sex, female % |
94 |
98 |
98 |
|
Age <65 years % |
76.9 |
81.1 |
|
|
Years since |
7.9 |
8.3 |
9.5 |
|
Mean (±SD) |
321.3 (121.9) |
323.1 (198.6) |
335.8 (187.9) |
|
ALP >3 x ULN % |
39.8 |
37.7 |
42.2 |
|
Mean (±SD) baseline total bilirubin mg/dL |
0.57 |
0.55 |
0.64 |
|
Mean PBC WI-NRS score |
3.3 |
3.2 |
|
|
Mean liver stiffness kPa |
9.9 |
10.7 |
|
|
Liver stiffness |
34 |
38 |
31 |
~41% of patients had moderate-to-severe pruritus3
~35% started the trial with advanced disease1,3,b
First second-line PBC treatment in which 13x more patients achieved biochemical response vs UDCA alone2
Biochemical response at 52 weeks2:
ALP <1.67 x ULN
ALP decrease ≥15% from baseline
Total bilirubin ≤ ULN
Biochemical response at week 522,c,d



cALP values are based on a weighted average of the ULN; 105 U/L is the weighted ULN based on a ULN of 129 U/L for men and 104 U/L for women.2
dSix patients in the IQIRVO group took IQIRVO alone, while 2 patients in the UDCA group took placebo alone.2
Helen, age 42
Disease history
- 24 months since diagnosis
- Treated with UDCA (900 mg daily) for 24 months
- Current ALP 218 U/L (2.1 x ULN) vs 353 U/L (3.4 x ULN) at diagnosis4
- 220 U/L ALP at 6-month marker vs 240 U/L at 12 months after starting therapy
- Current bilirubin 0.56 mg/dL (normal) vs 0.56 mg/dL (normal) at diagnosis5
Risk factors for progression
- Inadequate response to first-line treatment6
- Age <45 at diagnosis6,7
ALP levels with first-line treatment


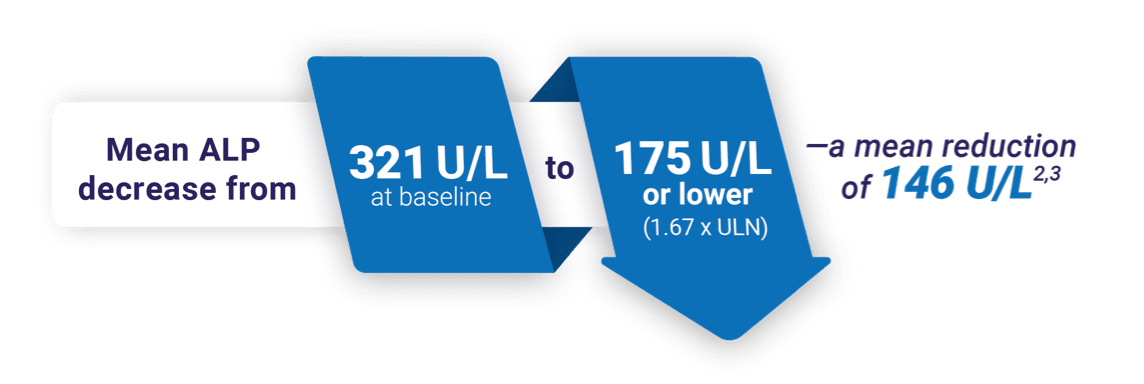
With IQIRVO, 15% of patients were able to achieve ALP normalization of 105 U/L or lower. Lowering ALP is a key treatment goal in managing PBC2,8
Hear key opinion leaders Dr. Kowdley, Dr. Kumar, and Dr. Flamm discuss biochemical response and normalizing ALP with IQIRVO
Hear key opinion leaders
Dr. Kowdley, Dr. Kumar, and Dr.
Flamm discuss biochemical
response and normalizing ALP
with IQIRVO
71% of patients in a subgroup analysis saw biochemical response1
In patients with ALP ≤3 x ULN at baseline
Biochemical response at week 521,c


|
ALP1 |
IQIRVO |
UDCA |
|---|---|---|
|
≤2 x ULN |
87% |
13% |
|
>2 – ≤2.5 x ULN |
80% |
0% |
|
>2.5 – ≤3 x ULN |
52% |
0% |
In patients with ALP >3 x ULN at baseline
21% (n=9/43) of patients saw biochemical response1
Risk difference: 21% (95% CI: 2% to 35%)
In patients with ALP >3 x ULN at baseline
21% (n=9/43) of patients saw biochemical response1
Risk difference: 21% (95% CI: 2% to 35%)
cALP values are based on a weighted average of the ULN; 105 U/L is the weighted ULN based on a ULN of 129 U/L for men and 104 U/L for women.2
Marie, age 57
Disease history
- 5 years since diagnosis
- Second-line treatment added 6 months ago, in combination with UDCA (prescribed at diagnosis)
- 5 years (UDCA), 1.5 years (UDCA + obeticholic acid)
- Recently reported symptoms of increased pruritus
- Current ALP of 239 U/L (2.3 x ULN) vs 187 U/L (1.8 x ULN) at diagnosis4
IQIRVO could help patients like Marie achieve biochemical response with an established safety profile2
Review safety and tolerability findings for IQIRVO.
Rapid ALP reduction as early as 4 weeks, sustained over 3 years1
ALP reduction from baseline1



37% reduction in ALP
as early as 4 weeks with IQIRVO1
37% reduction in ALP
as early as 4 weeks with IQIRVO1
ALP reduction across subgroups Powerful ALP reduction, regardless of starting point1
Mean change from baseline to week 52 in ALP,
stratified by baseline ALP levels1


Data are presented descriptively.
A 22x greater reduction in ALP with IQIRVO vs UDCA alone3
LS mean change from baseline: IQIRVO -117 U/L, UDCA -5.3 U/L3
Normalization at 52 weeks in a pivotal study2
Target an ALP of 105 U/L or lower—only with IQIRVO2,c
ALP normalization at week 522



(n=108)(n=53)

cALP values are based on a weighted average of the ULN; 105 U/L is the weighted ULN based on a ULN of 129 U/L for men and 104 U/L for women.2
Data on reduction of pruritus with IQIRVO3
IQIRVO was studied in patients with moderate-to-severe pruritus, defined as a PBC WI-NRS score ≥4. The change in pruritus from baseline through weeks 24 and 52 were secondary endpoints of the study3
Results with IQIRVO presented below were not statistically significant and, therefore, these results are descriptive only.3
A trend toward improving pruritus was observed with IQIRVO, as measured by WI-NRS (LS mean change from baseline -1.93 vs -1.15 with UDCA alone; 95% CI: -2.0 to 0.4).3
Pruritus impact vs UDCA alone was measured by PBC-40 and 5-D itch scales3,d
PBC-40 itch domain at week 523


The PBC-40 is a patient-derived measure validated for PBC, covering 6 domains: fatigue, pruritus, cognitive, emotional, social, and other symptoms.9
A 0.5-point change per question in the itch domain was a threshold for response, with ≥1.5 change being clinically meaningful.9,10
5-D itch score at week 523
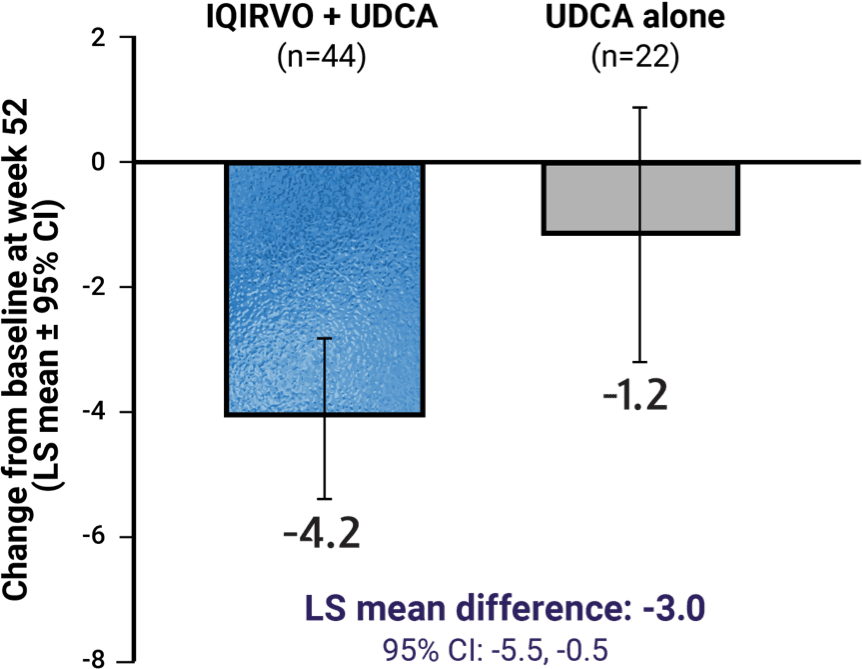

The 5-D itch scale is a patient-reported measure of the degree, duration, direction, disability, and distribution of pruritus.11
dSix patients in the IQIRVO group took IQIRVO alone, while 2 patients in the UDCA group took placebo alone.2
Data on reduction of fatigue and excessive sleepiness with IQIRVO1
- An analysis of fatigue and sleep via the PRO Measurement Information System (PROMIS) Fatigue Short Form 7a (PROMIS fatigue), PBC-40 fatigue domain, and Epworth Sleepiness Scale (ESS) was performed with patients who completed the ELATIVE double-blind period and entered the OLE1
- Changes in fatigue/sleepiness were summarized from baseline to week 104 and week 130 with respect to available MCID, categorical changes, and mean change from baseline in total score1
The data are from a single arm of the OLE study, including only patients on IQIRVO during ELATIVE. No formal statistical analysis was conducted, and results are descriptive only.
Mean change from baseline in total score of the PROMIS fatigue, PBC-40 fatigue, ESS1,e



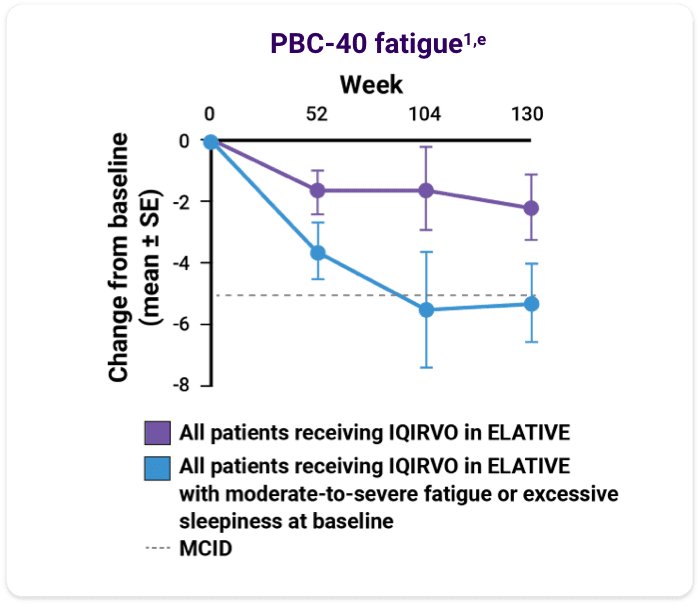

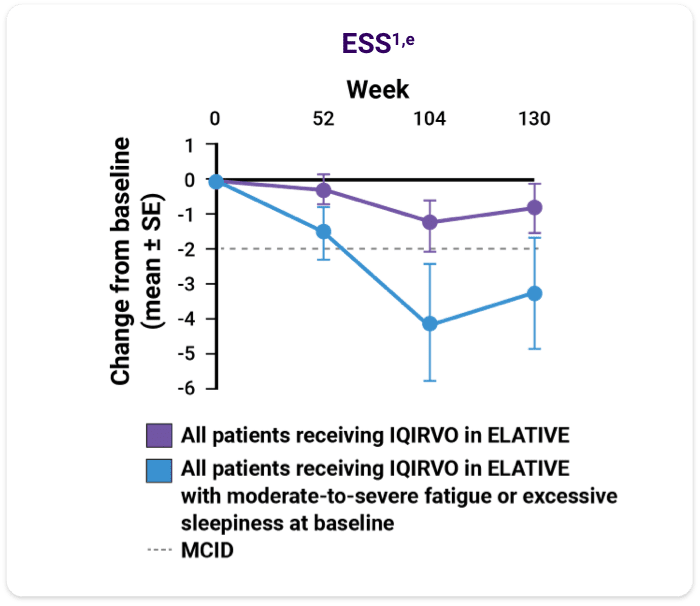
ePatients included are those with non-missing data at baseline and each time point of interest. Moderate-to-severe fatigue was defined as a PROMIS fatigue total score ≥60 or PBC-40 fatigue domain total score ≥29 at baseline. Week 52: n=95, week 104: n=48, week 130: n=26. Excessive sleepiness was defined as ESS total score ≥10 at baseline. Dotted lines reflect MCID for each measure.1
aPatients completing the ELATIVE double-blind period were eligible to enter the OLE trial to receive IQIRVO 80 mg daily. Baseline in this OLE crossover arm was defined as the last available measurement before the first OLE IQIRVO dose.1
bLiver stiffness >10 kPa and/or bridging fibrosis or cirrhosis on histology.1
ALP=alkaline phosphatase; CI=confidence interval; LS=least squares; MCID=minimal clinically important difference; OLE=open-label extension; PBC=primary biliary cholangitis; SD=standard deviation; SE=standard error; UDCA=ursodeoxycholic acid; ULN=upper limit of normal; WI-NRS=worst itch numeric rating scale.
References: 1. Data on file. Ipsen Biopharmaceuticals, Inc. 2. IQIRVO [package insert]. Ipsen Biopharmaceuticals, Inc. Cambridge, MA. 3. Kowdley KV, Bowlus CL, Levy C, et al; ELATIVE Study Investigators’ Group. Efficacy and safety of elafibranor in primary biliary cholangitis. N Engl J Med. 2024;390(9):795-805, suppl. 4. ALKP. Mayo Clinic Laboratories. Accessed February 6, 2025. https://www.mayocliniclabs.com/test-catalog/overview/622157#Clinical-and-Interpretive. 5. BILI3. Mayo Clinic Laboratories. Accessed February 6, 2025. https://www.mayocliniclabs.com/test-catalog/overview/8452#Clinical-and-Interpretive. 6. Hirschfield GM, Chazouillères O, Cortez-Pinto H, et al. A consensus integrated care pathway for patients with primary biliary cholangitis: a guideline-based approach to clinical care of patients. Expert Rev Gastroenterol Hepatol. 2021;15(8):929-939. 7. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67(1):145-172. 8. Kowdley KV, Bowlus CL, Levy C, et al. Application of the latest advances in evidence-based medicine in primary biliary cholangitis. Am J Gastroenterol. 2023;118(2):232-242. 9. Jacoby A, Rannard A, Buck D, et al. Development, validation, and evaluation of the PBC-40, a disease specific health related quality of life measure for primary biliary cirrhosis. Gut. 2005;54(11):1622-1629. 10. Jones D, Carbone M, Invernizzi P, et al. Impact of setanaxib on quality of life outcomes in primary biliary cholangitis in a phase 2 randomized controlled trial. Hepatol Commun. 2023;7(3):e0057. 11. Elman S, Hynan LS, Gabriel V, et al. The 5-D itch scale: a new measure of pruritus. Br J Dermatol. 2010;162(3):587-593.


